Systemic Lupus Erythematosus
- Non-communicable
- Autoimmune
- Systemic
Systemic lupus erythematosus (SLE) is an autoimmune disease that is caused by chronic inflammation and tissue damage due to autoreactive antibodies. The major pathogenic cellular characteristic is abnormal B cell activity. The secreted auto-reactive antibodies are directed toward cellular components and can cause multiorgan damage by immune complex deposition and inflammation within affected tissues. Hereditary mutations in human leukocyte antigen (HLA) genes further contribute to the dysregulated signaling between T helper cells and B cells. SLE is most prevalent in adult women, while various genetic and environmental factors play a role in the disease process. Non-specific SLE can vary from systemic symptoms to musculoskeletal or dermatological features. Clinical features are reflected both in manifestations of active lupus, caused by inflammation events, and chronic organ damages. There are various therapeutic medications for SLE that can prevent flares, as well as reduce disease severity and duration. Currently used treatment methods include disease-modifying antirheumatic drugs (DMARDs), immune cell targeted therapies and anti-cytokine medications.
Clinical Features
The autoimmune disease SLE is characterized by various symptoms that can affect any part of the body with commonly occurring relapses and remission. The clinical manifestations can be subdivided into two main groups: active lupus features caused by inflammation events and secondly, chronic damage. Symptoms can vary from systemic clinical features to musculoskeletal or dermatological features. Since the symptoms and comorbidities cover a wide variation, they are not specific for SLE. Therefore, diagnosis remains a huge challenge in hospitals and led to SLE commonly being referred to as “The great imitator”, since it mimics many other illnesses. Nonetheless, with differential diagnosis methods in 104 of 130 cases (80 %) patients with at least one clinical criterion are diagnosed positive correctly.
Clinical diagnosis and monitoring of SLE is facilitated by the clinical disease activity index (SLEDAI). The standardized indices are useful for assessing the SLE disease activity of certain features and weighing their importance. Typical laboratory methods are the measurement of SLE-specific anti-dsDNA or anti-C1q antibodies from blood specimens. Systemic chronic complaints that are not part of suggestive diagnosis are fever, hair loss, oral ulcers, photosensitivity,
malaise
Malaise
General feeling of discomfort or unease, usually an early indicator for other symptoms to follow.
, joint pain, fatigue. 70 % of all SLE patients suffer from severe skin problems. Since the skin is the main systemically affected organ, three different lesion types are distinguished between the patients. First chronic cutaneous lupus, second subacute cutaneous lupus, third acute cutaneous lupus. The first group is likely to exhibit thick, red scaly patches, whereas the patches of the second group have distinct edges. The third type is characterized by butterfly rashes. Furthermore, muscle pain is commonly reported in 90% of all SLE patients.
The patients suffer from joint pain in small hand and wrist joints, but in contrast to arthritis the joints are not destructed. Another typical feature is the proof of blood and proteins in the urine. Especially for patients with lupus nephritis a severe renal impairment and kidney failure is diagnosed. The histological hallmark in this context is membranous glomerulonephritis, which is caused by the deposition of immune complexes along the glomerular base membrane. Inflammation events can spread to the lung, which leads to inflammation of the pleurae and finally, to diminished lung volume. Pneumonitis, pulmonary hypertension and emboli can occur as well.
Many neurological diseases that affect the central or peripheral nervous system count as indicative factors for SLE and increase the mortality rate immensely. Complaints like headache, cognitive dysfunction, mood disorder, polyneuropathy, psychosis are common symptoms. Frequently the damaged epithelium of the blood-brain barrier is inducing brain-associated symptoms. SLE can affect the reproductivity of women, which is confirmed by spontaneous abortion and live-birth rates of only 72%.
Pathogenesis
Apoptosis
One of the major disease-causing mechanisms of SLE is the defective clearance of dead cells. The consequence of dysregulated apoptotic clearance leads to the presence of necrotic cells. These dead cells are not processed by macrophages. Instead they engulf self-antigens by exposing blebs on their membrane surface. The presented intracellular proteins represent auto-antigens, which provoke the secretion of inflammatory cytokines and IFN-α. Furthermore TLR7/9 is activated through presented DNA fragments, which enhances the production of IFN by dendritic cells. This overload of IFN results in chronic inflammation.
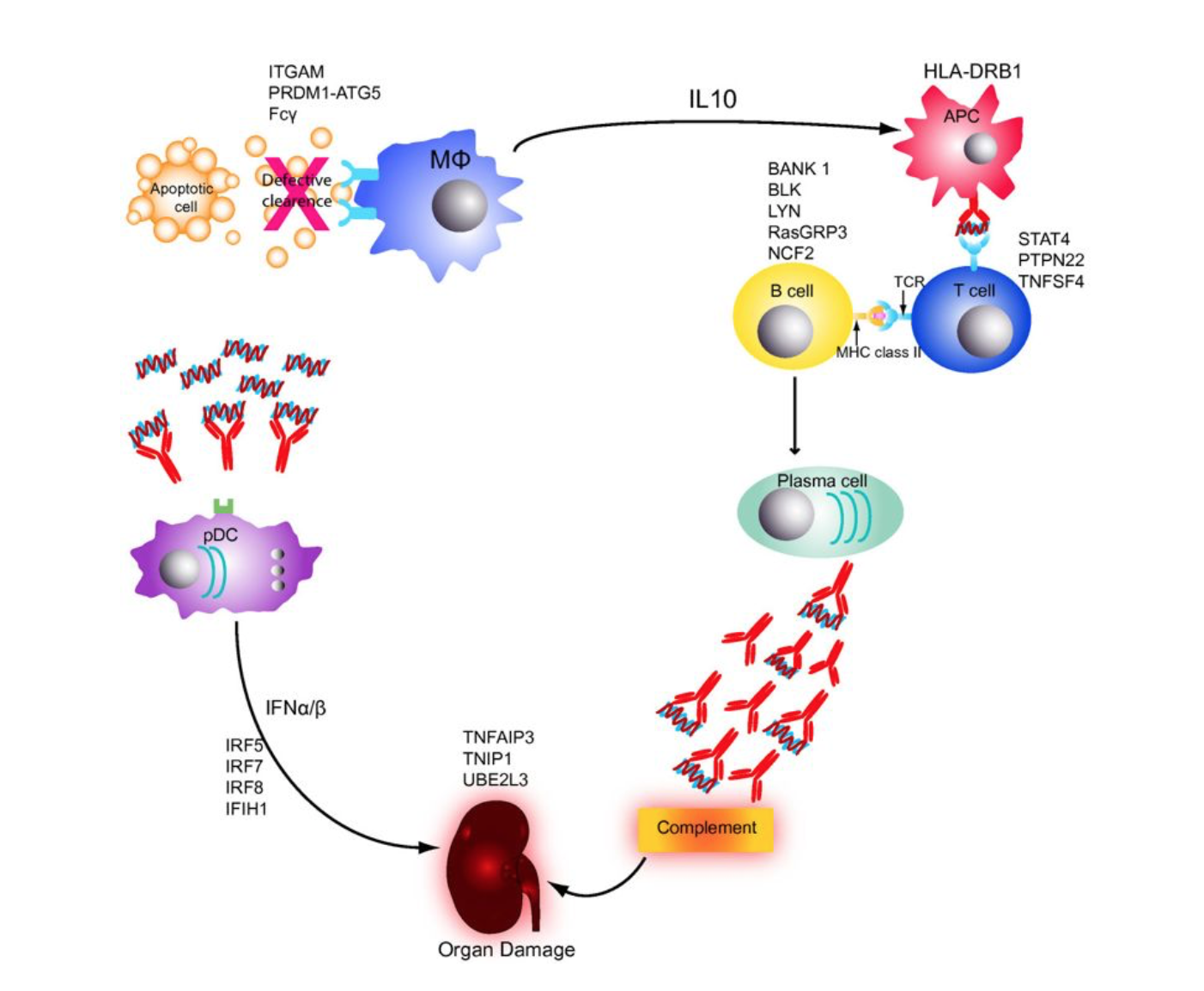
Figure 1: The impaired immune system in patients with systemic lupus erythematosus. Adopted from Guerra et al.
The
antigen
Antigen
A structural molecule that is recognised by the adaptive immune system, binding to a T cell receptor (TCR) or B cell receptor (BCR) or antibody.
presentation with an MHC receptor to T cells leads to the maturation into T helper cells. The T helper cells can bind with their T cell receptor to the
antigen
Antigen
A structural molecule that is recognised by the adaptive immune system, binding to a T cell receptor (TCR) or B cell receptor (BCR) or antibody.
-MHC II complex on B cells. They maturate to auto-antibody secreting
plasma
Plasma
Blood plasma is the yellow-colored portion of the blood carrying cells and proteins throughout the body.
cells, which attack healthy tissue. The auto-antigens induce the loss of self-tolerance of T and B cells and enhance the secretion of auto-antibody production.
Ubiquitination
The regulative
protein
Protein
Large biomolecules, which consist of one or more long chains of amino acid residues. They perform a vast array of functions within organisms including transporting molecules, providing cellular structure and catalysising processes such as DNA replication.
A20 is downregulated in SLE patients, which leads to uncontrolled NFκB signaling. This results easily in impaired apoptotic mechanisms and chronic inflammation. This enzyme has a huge effect on ubiquitination mechanisms of proteins. Ubiquitin molecules that are attached to proteins are important for proteasome-dependent degradation. The post-translational modification also influences the termination of pro-inflammatory signaling pathways like NFκB, where the ubiquitination of IKKy is required for the release of NFκB. A20 regulates the receptor-interacting
protein
Protein
Large biomolecules, which consist of one or more long chains of amino acid residues. They perform a vast array of functions within organisms including transporting molecules, providing cellular structure and catalysising processes such as DNA replication.
kinase (RIP) and tumor necrosis factor receptor-associated kinase 6 (TRAF6) by ubiquitination. Mutations in the enzyme lead to impaired repression of NFκB activation by TNF induction and therefore to uncontrolled pro-inflammatory events.
Comorbidities
Viral or bacterial infections can both trigger or coexist with SLE and are the most common death cause associated with SLE. Less than 10 % develop deformities of hands and feet. A higher risk of osteoarticular arthritis has been reported for SLE patients. Rheumatoid arthritis and bone fractures are frequently accompanying the disease. Another common comorbidity is anemia in 50% of the SLE cases. Apart from the decreased numbers of red blood cells and haemoglobin level, also the white blood cells levels are lower in SLE patients. An association with the antiphospholipid antibody syndrome is documented, which is manifested in a prolonged partial thromboplastin time. Involvement of the heart can result in pericarditis, myocarditis or non-infectious endocarditis. Premature arteriosclerosis is another common complication of SLE
Cellular Characteristics
Lymphocytes
An abnormal lymphocyte differentiation process has been reported in SLE patients. The transcription factor ETS1 is dysregulated and impairs the proper B cell differentiation and Th17 proliferation. This pathogenic trait is considered to contribute to more inflammation events by abnormal B cell differentiation into immunoglobulin-secreting
plasma
Plasma
Blood plasma is the yellow-colored portion of the blood carrying cells and proteins throughout the body.
cells.
B cells
The major pathogenic cellular characteristic is abnormal B cell activity. An inability to clear apoptotic cells leads to the loss of self-tolerance in B cells. In SLE patients hyperactive B cells that are producing auto-antibodies are the most specific disease trait. The antibodies are directed towards cellular and nuclear components and cause multiorgan damage by immune complex deposition and inflammation within affected tissues. B cells are likely to activate CD4+ T helper cells (Th1/Th2), they inhibit regulatory T cells and induce elevated secretion of cytokines. Possible genetic contributors are the proteins LYN and BANK1. LYN kinase has a huge effect on inhibiting CD22 signaling. The downregulated expression of LYN in SLE causes hyper-responsive B cells. As described previously, the clearance deficiency is another triggering factor that causes autoreactive B cell maturation due to APC and T cell-based activation.
T cells
Apart from B cells also T cells such as CD3+CD4−CD8− T cells and Th17 cells are over-representative in the blood of SLE patients. This overload of lymphocytes immensely influences B cell interactions and tissue damage. One cellular cause for hyperactive T cells is the high CD3-TCR exposure, which leads to increased expression of CD40L and of cAMP-responsive element modulator (CREM). The overexpression of the transcription factor STAT4 is responsible for the development of naïve CD4+ cells to Th1 cells. Furthermore, it enhances the release of IFN-γ, which can result in chronic inflammation in organs such as the kidneys. Another stimulating mechanism is the OX40L/OX40 interaction between
antigen
Antigen
A structural molecule that is recognised by the adaptive immune system, binding to a T cell receptor (TCR) or B cell receptor (BCR) or antibody.
presenting cells (dendritic cells, B cells) and activated CD4+ and CD8+ T, which supports T cell survival. The high number of active T cells builds the base for the activation of B cells that can mature into
plasma
Plasma
Blood plasma is the yellow-colored portion of the blood carrying cells and proteins throughout the body.
cells secreting damaging auto-antibodies.
Human Leukocyte Antigens
One of the most important pathogenic factors is the genetic association of MHC genes. In particular, HLA class II receptors such as HLA-DRB1, HLA-DQA1 are expressed improperly in SLE patients. This impairs the
antigen
Antigen
A structural molecule that is recognised by the adaptive immune system, binding to a T cell receptor (TCR) or B cell receptor (BCR) or antibody.
presentation on APCs towards T cells. Apoptotic clearance deficiencies trigger the presentation of auto-antigens on MHC II receptors of
antigen
Antigen
A structural molecule that is recognised by the adaptive immune system, binding to a T cell receptor (TCR) or B cell receptor (BCR) or antibody.
presenting cells. The MHC mutations can therefore strengthen the interaction between APCs and T cells. Aside from HLA class II, HLA class III complex molecules such as C4A, C4Band C2 are also considered possible risk factors. These proteins play an important role in the activation of the complement pathway.
Interferons
Another frequently reported pathogenic feature is the dysregulated expression of genes that are involved in IFN pathways. Type I IFNs (IFN-α and IFN-β) are responsible for the activation and repression of T and B cells by mediating the Th1 response and controlling the B cell activation threshold. The induction of these immune cells results in chronic inflammation and possible tissue damage due to the (over)secretion of pro-inflammatory cytokines.
Genetics
Human Leukocyte Antigen
Genetic regions that are coding for HLAs are considered to be one of the major genetic causes underlying the manifestation of SLE. Major Histocompatibility Complex (MHC) molecules play an important role for the acquired immune response. Human MHC molecules are also called human leukocyte antigens (HLA) receptors that are presenting foreign or altered, endogenous cell proteins on the cell surface in order to bind T cells which activate the immune response cascade. MHC class II molecules are expressed on many
antigen
Antigen
A structural molecule that is recognised by the adaptive immune system, binding to a T cell receptor (TCR) or B cell receptor (BCR) or antibody.
presenting cells (APCs) and are responsible for the presentation of extracellular proteins, which get into the cell by endocytosis. These epitopes from the antigen processing are the displayed part on the APCs and on B cells.
Since HLA genes are inherited, many HLA proteins are associated with the development of SLE. Therefore, characterisation of the corresponding chromosome regions can help to get a reliable diagnosis of autoimmune diseases. Based on current research HLA-DR2 and -DR3 have the strongest associations with SLE. Some studies have also shown associations between SLE and HLA-B7, -B18, -DQA1, -DQB1, -DQ3 and -DR7.
For Northern Europeans, DR3 shows a linkage disequilibrium with A1- Cw7-B8-TNFB*1-C2C-Bfs-C4AQ0-C4B1-DRB1*0301-DQA1* 0501-DQB1*0201. This event describes the close location of two different HLA loci on the same chromosome. This closeness can lead to the fact that some B and C alleles are expressed co-dominantly with a higher frequency than other combinations of codominance. Apart from HLA class II also HLA class III complex molecules such as C4A, C4B and C2 are considered as possible risk factors. These proteins play an important role in the activation of the complement pathway, which is crucial for the unspecific immune response. Partial or complete deficiencies in the
protein
Protein
Large biomolecules, which consist of one or more long chains of amino acid residues. They perform a vast array of functions within organisms including transporting molecules, providing cellular structure and catalysising processes such as DNA replication.
expression can result in SLE developing in early ages accompanied by renal diseases. Significant increase of C4Anull alleles among African SLE patients (61%, P < 0.001) and European versus Americans (43%, P < 0.01) has been reported.
The rate of C2 deficiencies is elevated in lupus patients with 0.4–2% compared to 0.01% in the non-lupus affected population. Impaired C2 expression is associated with cutaneous and articular involvement and neurologic or renal involvement. Higher photosensitivity and higher levels of anti-Ro auto-antibodies are common. 91 % of all individuals with a C1q deficiency are diagnosed as SLE patients. This HLA
protein
Protein
Large biomolecules, which consist of one or more long chains of amino acid residues. They perform a vast array of functions within organisms including transporting molecules, providing cellular structure and catalysising processes such as DNA replication.
leads to an excessive prevalence of the disease and leads to severe illness.
Genes on the X chromosome
Many other genes have been identified as possible risk factors for SLE. Some of the hereditary prevalence is population dependent. A total familiar hereditability of > 66 % for SLE is reported.
The prevalence of SLE is significantly higher for women, which is mainly caused by variations of two genes on chromosome X, interleukin-1 receptor-associated kinase 2 (IRAK2) and methyl CpG binding
protein
Protein
Large biomolecules, which consist of one or more long chains of amino acid residues. They perform a vast array of functions within organisms including transporting molecules, providing cellular structure and catalysising processes such as DNA replication.
2 (MECP2). The kinase IRAK 1 is involved in the signaling cascade of the Toll/IL-1 receptors and has also a huge effect on the activation of the NFκB signaling. Also, IFN-regulated genes are overexpressed in dysregulated B cells due to elevated induction of IFN-α and IFN-γ. MECP2 binds to methylated DNA and induces the histone deacetylase. It is also responsible for the activation or repression of genes with CG-rich promoter sequences.
Other associated genes
Another SLE associated genetic risk factor is TNFSF4 (OX40L), which is likely to be overexpressed in SLE patients. The
protein
Protein
Large biomolecules, which consist of one or more long chains of amino acid residues. They perform a vast array of functions within organisms including transporting molecules, providing cellular structure and catalysising processes such as DNA replication.
is expressed on
antigen
Antigen
A structural molecule that is recognised by the adaptive immune system, binding to a T cell receptor (TCR) or B cell receptor (BCR) or antibody.
presenting cells and interacts with the T cell specific OX40 receptor. The interaction between OX40L and OX40 results in increased survival rates of activated T cells. The increased binding events in SLE patients and the consequent T cell activation contributes to the
pathogenesis
Pathogenesis
The biological mechanism that leads to a state of disease. It can refer to the origin and development of a disease, as well as whether it is recurrent, chronic and acute.
of the disease.
The promoter of the pentraxin C-reactive
protein
Protein
Large biomolecules, which consist of one or more long chains of amino acid residues. They perform a vast array of functions within organisms including transporting molecules, providing cellular structure and catalysising processes such as DNA replication.
(CRP) gene is prone to be expressed differently in Caucasian and African ethnicities. CRP has a great impact on the clearance of apoptotic bodies. The defective clearance mechanism enhances the creation of autoantibodies.
More dysregulated genes associated with SLE are interferon regulating factor 5 (IRF5) and PTPN22. IRF5 is controlling the inhibition or activation of inflammatory actions of macrophages, whereas the lymphoid phosphatase PTPN22 affects the responsiveness of B/T cell receptors. Furthermore, the transcription factor STAT4 is prone to be overexpressed in SLE patients, which leads to enhanced maturation of naive CD4+ T cell to Th1 cells.
Treatment
Many different therapeutic medications are approved for clinical use. The treatment of SLE promises the prevention of flares and the reduction of disease severity and duration. The currently used treatment methods can be subdivided into three major groups: antirheumatic drugs, immune cell targeted therapies and anti-
cytokine
Cytokine
A broad category of small proteins released by a range of cells (including immune cells like macrophages, B lymphocytes, T lymphocytes) that act through receptors and are important for a range of processes including cell signaling, inhibition, maturation and proliferation.
medications.
Disease modifying antirheumatic drugs (DMARDs)
Antimalarial hydroxychloroquine is mainly used to treat mild symptoms of SLE or to prevent effectively the worsening of new mild manifestations. It is not effective against severe cases of SLE and it’s not suitable for pregnant women. The mode of action is based on the inhibition of phagosomes, which is achieved by downregulating the TLR activation on dendritic cells and consequently the IFN-α release. The
dendritic cell
Dendritic Cell
Cells that function as a conduit between the innate and adaptive immune systems.
maturation is impaired and nuclear antigens cannot be presented to T cells.
Corticosteroids are commonly given to SLE patients at the beginning of flares. It has a strong anti-inflammatory effect by impairing both the acquired and the innate immune response. The immunosuppressive effect is mainly based on the inhibition of B and T Cell activation and secondly on the impaired NFκB pathway and consequently impaired effector functions of monocytes and neutrophils. They are administered orally, only in cases where > 60 mg are required the intravenous pulse therapy is applied.
Cyclophosphamide (CTX) is likely to be used for patients with lupus nephritis and in cases where other DMARDs are ineffective. The drug leads to cell apoptosis. Due to many possible side effects like malignancy and infections, immunosuppressive drugs such as mycophenolic acid are preferred.
Mycophenolate mofetil is the prodrug of mycophenolic acid, which inhibits the inosine mono phosphate dehydrogenase. The mode of action is based on controlling the de novo synthesis of guanosine nucleotides and the following DNA synthesis in lymphocytes. The active metabolite inhibits the purine synthesis in proliferating B and T cells. It has been reported to be more effective than azathioprin and CTX, but the treatment is commonly accompanied by gastrointestinal side effects.
Azathioprine (AZA) is a purine analogue that is metabolized to the active mercaptopurine in the liver. The inhibitory effect on the DNA synthesis impairs the proliferation of immune cells. The side effects affect the gastrointestinal tract and can lead to oral ulcers, nausea and dose-dependent anemia and leucopenia.
Methotrexate (MTX) is an analogue of folic acid and acts as a competitive inhibitor of the enzyme dihydrofolate reductase. The inhibition results in impaired RNA and DNA synthesis. Clinical reports have shown that MTX is effective against SLE associated symptoms like arthritis and skin problems. It is rather ineffective if severe organ involvement occurs during the illness.
Immune cell targeted therapies
Anti-CD20 (rituximab) is a chimeric mouse-human antibody against B cell specific CD20 receptors. Rituximab is effectively used against many autoimmune diseases such as rheumatoid arthritis, multiple sclerosis and SLE. The antibody starts to act at the pre-B cell stage in the bone marrow until the mature naïve and memory B cell stage. B- cells are completely depleted from the peripheral blood, whereas
plasma
Plasma
Blood plasma is the yellow-colored portion of the blood carrying cells and proteins throughout the body.
cells and the immunoglobulin level remain the same. The drug has a high safety level and shows an effective response up to 12 months.
Anti-CD22 (epratuzumab) is a monoclonal antibody that is reactive against CD22 receptors. CD22 is an important B cell
antigen
Antigen
A structural molecule that is recognised by the adaptive immune system, binding to a T cell receptor (TCR) or B cell receptor (BCR) or antibody.
that is crucial for B cell receptor (BCR) signaling. Epratuzumab decreases the level of B cells up to 35 %, whereas immunoglobulin levels are not attacked.
Abetimus sodium (LJP-394) is a B cell tolerogen that is binding and crosslinking membrane associated immunoglobulins. This effect results in either anergy or complete deletion of reactive BCRs. Abetimus consists of four strands of dsDNA bound to a carrier, which binds the anti-dsDNA antibodies. The DNA
epitope
Epitope
A part of an antigen that is recognised by the immune system, specifically by antibodies, B cells, or T cells.
of the drug has a strong affinity to the patient’s antibodies. The drug is effective against renal flares.
Belimumab is a full human monoclonal antibody that reacts against the soluble B lymphocyte stimulator (BlyS). Consequently, BlyS cannot bind TAI, BCMA, BAFF receptors, which are elevated in SLE patients. The clinical efficacy and high tolerance in phase II and III studies have already been proven. Treated patients showed a decreased level of B cells and low titers of anti-dsDNA IgG, IgA, IgM and IgG.
Anti-cytokine therapies
This type of therapy is commonly used for SLE patients, since the illness is associated with dysregulated
cytokine
Cytokine
A broad category of small proteins released by a range of cells (including immune cells like macrophages, B lymphocytes, T lymphocytes) that act through receptors and are important for a range of processes including cell signaling, inhibition, maturation and proliferation.
secretion mechanisms, which are crucial for the regulation of the immune system.
TNF is a pleiotropic
cytokine
Cytokine
A broad category of small proteins released by a range of cells (including immune cells like macrophages, B lymphocytes, T lymphocytes) that act through receptors and are important for a range of processes including cell signaling, inhibition, maturation and proliferation.
that can promote or decrease autoimmunity. Since TNF levels are likely to be elevated in SLE patients, the blocking of TNF expression or release is considered as an effective and safe way of treatment. In this field many preclinical and clinical studies are ongoing.
IL-10 is overexpressed in the macrophages, dendritic cells and helper T cells of SLE patients. Actions of IL-10 on immune cells include the inhibition of both
macrophage
Macrophage
A variety of white blood cell which serves the primary function of digesting cellular debris.
and T cell activation and of pro-inflammatory
cytokine
Cytokine
A broad category of small proteins released by a range of cells (including immune cells like macrophages, B lymphocytes, T lymphocytes) that act through receptors and are important for a range of processes including cell signaling, inhibition, maturation and proliferation.
production. In contrast, IL-10 increases B cell survival, proliferation, differentiation and antibody production. The range of actions relevant to SLE are poorly understood and are still in the focus of SLE research. Pilot studies with mouse anti-IL-10 mAb (B-N10) are ongoing.
IFN-α is elevated in the serum of SLE patients. This
cytokine
Cytokine
A broad category of small proteins released by a range of cells (including immune cells like macrophages, B lymphocytes, T lymphocytes) that act through receptors and are important for a range of processes including cell signaling, inhibition, maturation and proliferation.
induces B cells in an IFN-α dependent way and leads to the generation of
plasma
Plasma
Blood plasma is the yellow-colored portion of the blood carrying cells and proteins throughout the body.
cells. MEDI-545 is a human monoclonal antibody that is neutralizing IFN-α, which has shown effects in the amelioration of SLE symptoms in clinical studie
Epidemiology
SLE occurs worldwide in all continents across various ethnical groups but with different distributions and incidence rates. The number of globally affected patients is 20-70/100 000 persons per year. The different outcomes of the illness depend on various factors such as genetics, environmental, sociodemographic and methodological features. For example, the exposure intensity to the sun is claimed to have a great influence on the disease process. Clinical studies have shown that non-Caucasians are more likely to develop severe SLE illness leading to early mortality. Individuals of black ethnicity have the highest incidence and prevalence rates, whereas white ethnicities have the lowest values. This is reflected in the incidence rate of 160/100 000 persons per year for Afro-Caribbeans, who are affected more frequently and severely than Europeans. From a geographical point of view the highest incidence rate has been documented in North America and in France with 23/100 000 persons diagnosed per year. The lowest rates are observed in Africa, Ukraine and Australia with each 0.3/100 000 persons diagnosed per year.
SLE affects individuals from both genders, but with a higher prevalence in women. Females are approximately nine times more likely to be affected by SLE compared to males. Females are also more likely to develop relapses, low white cell numbers, arthritis and psychiatric symptoms. Male patients in contrast are more likely to show symptoms like seizures, kidney and skin associated issues. Studies have proven that the gender imbalance may be caused by the X chromosome, which is the main carrier of immunologic genes that can mutate during the disease process. The incidence of SLE commonly peaks in middle adulthood, with the average age of symptom onset 30 +/- 10 years. The rates of children affected are relatively low compared to adult incidence rates. Childhood-onset illness is more severe and the auto-destructive mechanisms cause more damage, which results in lower survival rates in young individuals suffering from SLE.
References
- "SLE: Clinical presentations. Smith, Gordon. Autoimmunity Reviews. Volume 10, Issue 1, November 2010, Pages 43-45."
- "Clinical Features of Systemic Lupus Erythematosus Patients Complicated With Evans Syndrome. Zhang, Wu. Medicine (Baltimore). 2016 Apr; 95(15): e3279."
- "Clinical criteria for systemic lupus erythematosus precede diagnosis, and associated autoantibodies are present before clinical symptoms. Heinlen, McClain. Arthritis & Rheumathology. Volume56, Issue7July 2007. Pages 2344-2351."
- "Epdemiology of SLE. Pons-Estel GJ, Ugarte-Gil MF, Alarcón GS. Expert Rev Clin Immunol. 2017 Aug;13(8):799-814."
- "The worldwide incidence and prevalence of systemic lupus erythematosus: a systematic review of epidemiological studies.Rees F, Doherty M, Grainge MJ, Lanyon P, Zhang W.Rheumatology (Oxford).2017 Nov 1;56(11):1945-1961"
- "Current and Novel Therapeutics in Treatment of SLE. Diamond et al. J Allergy Clin Immunol. 2011 Feb; 127(2): 303–314. "
- "Abnormal B Cell Development in Systemic Lupus Erythematosus. Karrar, Graham et al.Arthritis Rheumatol. 2018 Apr; 70(4): 496–507"
- "The Pathogenesis of Systemic Lupus Erythematosus. Choi, Kim, Kraft et al. Curr Opin Immunol. 2012 Dec; 24(6): 651–657. "
- "The genetics of lupus: a functional perspective. Guerra, Cunninghame, Graham et al. Arthritis Research Therapy. BioMed Central Ltd 2012. Published: 29 May 2012."
- "Disturbances of apoptotic cell clearance in systemic lupus erythematosus. Shao, Cohen et al. Arthritis Res Ther.2011; 13(1): 202. Published online 2011 Feb 28."
- "Cellular and molecular pathogenesis of systemic lupus erythematosus: lessons from animal models. Pathak, Mohan et al. Arthritis Res Ther. 2011; 13(5): 241. "
- "Genetic Factors Predisposing to Systemic Lupus Erythematosus and Lupus Nephritis. Ramos, Brown et al. Semin Nephrol. 2010 Mar; 30(2): 164–176. "